Hepatitis B Virus
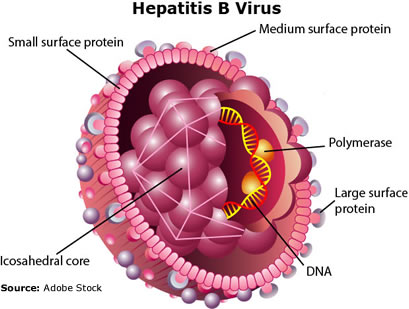 Hepatitis B is a vaccine-preventable, DNA genome, infectious viral disease (Thomas 2016). Hepatitis B virus (HBV) "belongs to the Hepadnaviridae family. It infects exclusively hepatocytes of humans and some non-human primates" (Herrscher 2020). HBV is responsible for significant morbidity and mortality in America and the world. HBV infections range from acute asymptomatic disease to chronic disease associated with cirrhosis and hepatocellular carcinoma. The CDC estimates about 862,000 people were living with HBV infections in the USA during 2016. During 2018, 1,649 U.S. death certificates reported HBV to be an underlying or contributing cause of death (CDC 2020).
Hepatitis B is a vaccine-preventable, DNA genome, infectious viral disease (Thomas 2016). Hepatitis B virus (HBV) "belongs to the Hepadnaviridae family. It infects exclusively hepatocytes of humans and some non-human primates" (Herrscher 2020). HBV is responsible for significant morbidity and mortality in America and the world. HBV infections range from acute asymptomatic disease to chronic disease associated with cirrhosis and hepatocellular carcinoma. The CDC estimates about 862,000 people were living with HBV infections in the USA during 2016. During 2018, 1,649 U.S. death certificates reported HBV to be an underlying or contributing cause of death (CDC 2020).
CDC Recommendations for HBV Screening
- People born in countries with an HBV prevalence of ≥2%
- People born in the United States not vaccinated as infants whose parents were born in regions with high rates of HBV infection (HBsAg prevalence of ≥8%)
- Men who have sex with men
- People who inject drugs
- People with HIV
- Household and sexual contacts of HBV-infected people
- People requiring immunosuppressive therapy
- People with end-stage renal disease (including hemodialysis patients)
- Blood and tissue donors
- People with elevated alanine aminotransferase levels (>19 IU/L for women and >30 IU/L for men)
- Screening of all pregnant women for HBsAg – HBV DNA testing for HBsAg-positive pregnant women, with suggestion of maternal antiviral therapy to reduce perinatal transmission when HBV DNA is >200,000 IU/mL
- Infants born to HBV-infected mothers (HBsAg and antibody to hepatitis B surface antigen [anti-HBs] only are recommended)
Prevention
- Standard Precautions (CDC 2016)
- Two therapies have been approved for HBV prevention: hepatitis B immune globulin (HBIG) for post-exposure prophylaxis (PEP) and hepatitis B vaccine.
- HBIG is prepared from plasma known to contain high concentrations of anti-HBs. The recommended dose of HBIG is 0.06 mL/kg body weight.
- Hepatitis B vaccine contains hepatitis B surface antigen (HBsAg) produced in yeast by recombinant DNA technology and provides protection from HBV infection when used for both pre-exposure prophylaxis (PrEP) vaccination and PEP. The three available monovalent hepatitis B vaccines for use in the United States are Recombivax HB, Engerix-B, and Heplisav-B. A combination hepatitis A and hepatitis B vaccine for use among persons aged ≥18 years, Twinrix, also is available.
- Vaccination
recommendations
- Universal vaccination of all infants beginning at birth, as a safeguard for infants born to HBV infected mothers not identified prenatally
- Routine vaccination of previously unvaccinated children aged <19 years
- Vaccination of adults at risk for HBV infection, including those requesting protection from HBV without acknowledgment of a specific risk factor.
Transmission
Hepatitis B (HBV) is transmitted through activities that involve percutaneous (i.e., puncture through the skin) or mucosal contact with infectious blood or body fluids (e.g., semen and saliva).
HBV incubation period
If symptoms occur, they begin an average of 90 days (range: 60–150 days) after exposure to HBV
Tests used to identify patients with hepatitis B?
Three different serologic tests are needed (hepatitis B surface antigen [HBsAg], hepatitis B surface antibody [anti-HBs], and total hepatitis B core antibody [anti-HBc]) to determine whether a patient
- has acute or chronic HBV infection and is in need of post-test counseling and linkage to care ,
- is immune to HBV as a result of prior infection or vaccination (in conjunction with vaccination history), or
- is susceptible to infection and in need of vaccination.
Treatment
Acute HBV - Therapy available to persons with acute HBV infection is supportive.
Chronic HBV - Persons with chronic HBV infection should be referred for evaluation to a provider experienced in managing such infections. Oral Antivirals (Nucleos(t)ide Analogues approved by FDA for treatment of chronic HBV infection can achieve sustained suppression of HBV replication and remission of liver disease.
| HBV vaccine |
An effective prophylactic vaccine against hepatitis B has been available for 40 years. However, no currative HBV treatment currently exist. |
| |
| Oral HBV antivirals |
Current FDA approved HBV antivirals are nucleoside reverse transcriptase inhibitors (NRTIs). These NRTIs competitively inhibit HBV DNA replication polymerase causing chain termination which stops viral replication. |
NRTIs |
Tenofovir disoproxil |
Entecavir |
Adefovir Dipivoxil |
| Tenofovir alafenamide |
Telbivudine |
Lamivudine |
| |
| Infusion |
|
Interferons |
Pegylated Interferon |
Pegylation involves the bonding of polyethylene glycol to a molecule, which can increase the size and reduce renal clearance, mask interferon from the immune systyem and provide water solubility to hydrophobic drugs.
|
| Interferon Alpha |
|
Not all people newly infected with HBV have symptoms, but for those that do, symptoms can include fatigue, poor appetite, stomach pain, nausea, and jaundice. For many people, hepatitis B is a short-term illness. For others, it can become a long-term, chronic infection that can lead to serious, even life-threatening health issues like cirrhosis or liver cancer. Risk for chronic infection is related to age at infection: about 90% of infants with hepatitis B go on to develop chronic infection, whereas only 2%–6% of people who get hepatitis B as adults become chronically infected. The best way to prevent hepatitis B is to get vaccinated (CDC..
The onset of HBV is usually gradual and the majority of infected persons recover within six months. However, 5 to 10% of patients with HBV infection develop chronic hepatitis or become carriers. Chronic hepatitis increases the risk of developing cirrhosis, primary hepatocellular carcinoma and fulminant hepatitis, which is almost always fatal. Chronic hepatitis is an inflammatory reaction of the liver that lasts for more than 6 months. Fulminant hepatic failure is massive liver cell death within 2 months of the development of acute hepatitis. Death occurs in 80% of patients with fulminant hepatic failure due to gastrointestinal bleeding, sepsis, brainstem compression from cerebral edema, or multisystem failure. People with HBV can also become carriers of the virus and can transmit it to others.
CDC estimates 850,000 to 2.2 million persons are living with HBV infection (prevalence) in the United States. Persons in high risk groups, should receive HBV vaccine which provides active immunity for up to 5 years. The Occupational Safety and Health Administration requires that health care facilities offer the HBV vaccine to those employees with jobs that require exposure to blood, blood products, or other potentially infectious materials.
References
MMWR (2018). Prevention of hepatitis B virus infection in the united ... . Retrieved October 28, 2021, from https://www.cdc.gov/mmwr/volumes/67/rr/pdfs/rr6701-H.PDF.
Centers for Disease Control and Prevention. (2020). Health Care Providers and viral hepatitis. Centers for Disease Control and Prevention. Retrieved October 28, 2021, from https://www.cdc.gov/hepatitis/populations/healthcaresettings.htm.
Centers for Disease Control and Prevention. (2020). Hepatitis B questions and answers for Health Professionals. Centers for Disease Control and Prevention. Retrieved October 26, 2021, from https://www.cdc.gov/hepatitis/hbv/hbvfaq.htm#ref03.
Centers for Disease Control and Prevention. (2016). Standard precautions for all patient care. Centers for Disease Control and Prevention. Retrieved October 27, 2021, from https://www.cdc.gov/infectioncontrol/basics/standard-precautions.html.
Herrscher, C., Roingeard, P., & Blanchard, E. (2020). Hepatitis B Virus Entry into Cells. Cells, 9(6), 1486. https://doi.org/10.3390/cells9061486
Schillie S, Vellozzi C, Reingold A, et al. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2018;67(No. RR-1):1–31. DOI: http://dx.doi.org/10.15585/mmwr.rr6701a1
Thomas, E., Liang, T. Experimental models of hepatitis B and C — new insights and progress. Nat Rev Gastroenterol Hepatol 13, 362–374 (2016). https://doi.org/10.1038/nrgastro.2016.37
© RnCeus.com
 Hepatitis B is a vaccine-preventable, DNA genome, infectious viral disease (Thomas 2016). Hepatitis B virus (HBV) "belongs to the Hepadnaviridae family. It infects exclusively hepatocytes of humans and some non-human primates" (Herrscher 2020). HBV is responsible for significant morbidity and mortality in America and the world. HBV infections range from acute asymptomatic disease to chronic disease associated with cirrhosis and hepatocellular carcinoma. The CDC estimates about 862,000 people were living with HBV infections in the USA during 2016. During 2018, 1,649 U.S. death certificates reported HBV to be an underlying or contributing cause of death (CDC 2020).
Hepatitis B is a vaccine-preventable, DNA genome, infectious viral disease (Thomas 2016). Hepatitis B virus (HBV) "belongs to the Hepadnaviridae family. It infects exclusively hepatocytes of humans and some non-human primates" (Herrscher 2020). HBV is responsible for significant morbidity and mortality in America and the world. HBV infections range from acute asymptomatic disease to chronic disease associated with cirrhosis and hepatocellular carcinoma. The CDC estimates about 862,000 people were living with HBV infections in the USA during 2016. During 2018, 1,649 U.S. death certificates reported HBV to be an underlying or contributing cause of death (CDC 2020).