Medical Treatment
 The goal of hepatitis
C virus (HCV) treatment is evolving. Just a few years ago the goal was to slow the progression of the disease. Today the goal is to obtain a sustained virological response (SVR). SVR is considered a cure and has been associated with decreases in all-cause mortality, liver-related death, the need for liver transplantation, hepatocellular carcinoma rates, and liver-related complications.
The goal of hepatitis
C virus (HCV) treatment is evolving. Just a few years ago the goal was to slow the progression of the disease. Today the goal is to obtain a sustained virological response (SVR). SVR is considered a cure and has been associated with decreases in all-cause mortality, liver-related death, the need for liver transplantation, hepatocellular carcinoma rates, and liver-related complications.
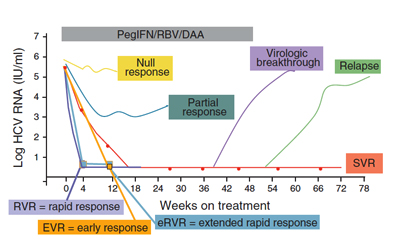
HCV nucleic acid tests to verify active infection, genotype and viral load are recommended to guide selection of the most appropriate antiviral regimen. The efficacy of treatment can be evaluated by HCV PCR assay. Declining HCV RNA during therapy is highly associated with the likelihood of achieving SVR. Attaining rapid virologic response (RVR), extended RVR (eRVR), and early virologic response (EVR) can provide guidance as to the likelihood of achieving an SVR. If there is no detectable amount
of HCV in the blood 6 months after completing a full course of treatment, then
SVR has been obtained and the treatment has worked.
The choice of treatment
may be influenced by infection status, including acute versus chronic infection and genotype. New treatment guidelines for patients with genotypes
2 or 3 recommend a daily oral ribavirin and a direct acting anti-viral regimen for treatment naive patients. Treatment-naive patients with HCV genotype 1 are recommended a regimen of daily oral direct acting anti-viral, ribavirin and weekly intravenous pegylated interferon.
Instant
Feedback:
The
goal of HCV anti-viral treatment is sustained virological response (SVR).
Chronic HCV Treatment•
"Until recently, the mainstay of treatment for chronic hepatitis C virus (HCV) infection has been pegylated interferon and ribavirin, with possible addition of boceprevir (Victrelis™) and telaprevir (Incivek™) (both protease inhibitors) for HCV genotype 1 infection. After given for 24-48 weeks, this treatment resulted in a sustained virologic response (a marker for cure), defined as undetectable HCV RNA in the patient's blood 24 weeks after the end of treatment in 50%–80% of patients (with higher SVR among persons with HCV genotypes 2 or 3 infections versus infections with HCV genotype 1, the most common genotype found in the United States)."
"In late 2013, The Food and Drug Administration approved two new direct acting antiviral drugs, Sofosbuvir (Sovaldi™) and Simeprevir (Olysio™) to treat chronic HCV infection. Both medications have proven efficacy when used as a component of a combination antiviral regimen to treat HCV-infected adults with compensated liver disease, cirrhosis, HIV co-infection, and hepatocellular carcinoma awaiting liver transplant. Clinical trials have shown that these new medications achieve SVR in 80%-95% of patients after 12-24 weeks of treatment."
"Sofosbuvir (Sovaldi™) is a nucleotide analogue inhibitor of the hepatitis C virus (HCV) NS5B polymerase enzyme, which plays an important role in HCV replication. It is taken orally once a day at a 400-mg dose. The drug is approved for two chronic hepatitis C indications: In combination with pegylated interferon and ribavirin for treatment-naïve adults with HCV genotype 1 and 4 infections, and in combination with ribavirin for adults with HCV genotypes 2 and 3 infection. The second indication is the first approval of an interferon-free regimen for the treatment of chronic HCV infection".
"Simeprevir (Olysio™) is a protease inhibitor that blocks a specific protein needed by the hepatitis C virus to replicate. It is to be used as a component of a combination antiviral treatment regimen of peginterferon-alfa and ribavirin for genotype 1 infections only. It is taken orally once a day at a 150-mg dose. The treatment duration is 24-48 weeks depending on prior treatment history and response to treatment. Because the efficacy of simeprevir is substantially reduced in patients infected with HCV genotype 1a with an NS3 Q80K polymorphism, screening for this mutation is strongly recommended by the manufacturer before treatment initiation."
Acute HCV Treatment:•
Acute HCV infection is defined as diagnosis within 6 months of exposure. During this period, it is believed that 20%-50% of untreated HCV infections clear spontaneously. Treatment for acute hepatitis C is similar to treatment for chronic hepatitis C. The response rate to treatment is higher among persons with acute than with chronic HCV infection. However, the optimal treatment regimen and when it should be initiated remains uncertain.
Treatment mechanisms and adverse events |
| Interferon |
Interferons are a group of proteins secreted by cells in response to pathogens or cancer. Infected cells secrete interferons to signal neighboring cells and the immune system to initiate protective defenses. Alpha interferon is an important part of the innate antiviral immune response. It is believed that alpha interferon binds to specific cell membrane receptors. Binding of alpha interferon to receptors stimulates gene activity that establishes an antiviral state within the target cells. When these interferon stimulated genes (ISG) are activated they block virion protein synthesis which inhibits virus replication and release. ISG can also induce the production of intracellular enzymes like RNAse that destroy viral RNA.
Unfortunately, the majority of HCV patients do not acheive SVR with interferon alone. HCV is particularly adept at working around the interferon defenses. "The high genetic variability of HCV allows the virus to passively evade the immune system. In addition, several viral genes impair cellular functions involved in immune response, or in cell proliferation, or cause apoptosis. Furthermore, HCV does not only infect hepatocytes but it infects B and T cells as well. Thus, infection of cells of the immune system impairs their functions." • |
Pegylated
Interferon |
Polyethylene glycol (PEG)
is a non-toxic polymer used
as the basis for a number of laxatives and skin creams.
When various protein medications
(such as interferon alpha) have PEG
attached, it provides a longer acting medicinal effect and/or reduces toxicity. |
| Ribavirin |
Ribavirin belongs to a
class of drugs called nucleoside analogues. This drug class includes some
anti-HIV drugs like zidovudine (AZT). Ribavirin is not effective used alone
for the treatment of Hepatitis C, but works with pegylated interferon. Together,
they are more effective preventing HCV from making new copies of itself, and
helping the immune system eliminate HCV. The dosage is
usually individualized according to body weight.
Ribavirin must not be
used in females who are pregnant, or males whose partners are pregnant,
because of toxicity that results in significant teratogenic and/or embryocidal
effects, even at very low doses. A negative pregnancy test must be obtained
immediately prior to treatment and females of child-bearing potential and
males must use 2 forms of birth control during treatment and for 6 months
after treatment. |
Boceprevir
(not recommended) |
Boceprevir is classified as a ketoamide protease inhibitor that inhibits HCV replication. These drugs bind to the active site of an HCV protease. Adverse events associated with boceprevir include anemia, dysgeusia , nausea, diarrhea and neutropenia. |
Telaprevir
(not recommended) |
Telaprevir is classified as a ketoamide protease inhibitor that inhibist HCV replication. These drugs bind to the active site of an HCV protease. Adverse events associated with telaprevir include the development of rash , anemia, hemorrhoids, anorectal discomfort, anal pruritus, dysgeusia, dizziness, depression, and hyperuricemia. |
Instant
Feedback:
Ribavirin must not be given to women who are pregnant, and women receiving
the drug must use double birth control.
©RnCeus.com
 The goal of hepatitis
C virus (HCV) treatment is evolving. Just a few years ago the goal was to slow the progression of the disease. Today the goal is to obtain a sustained virological response (SVR). SVR is considered a cure and has been associated with decreases in all-cause mortality, liver-related death, the need for liver transplantation, hepatocellular carcinoma rates, and liver-related complications.
The goal of hepatitis
C virus (HCV) treatment is evolving. Just a few years ago the goal was to slow the progression of the disease. Today the goal is to obtain a sustained virological response (SVR). SVR is considered a cure and has been associated with decreases in all-cause mortality, liver-related death, the need for liver transplantation, hepatocellular carcinoma rates, and liver-related complications.