 Vaccination is the most effective way to prevent influenza illness and associated complications. For the CDC SUMMARY of Prevention and Control of Influenza with Vaccines 2017–18 Influenza Season.
Vaccination is the most effective way to prevent influenza illness and associated complications. For the CDC SUMMARY of Prevention and Control of Influenza with Vaccines 2017–18 Influenza Season. Immunization
 Vaccination is the most effective way to prevent influenza illness and associated complications. For the CDC SUMMARY of Prevention and Control of Influenza with Vaccines 2017–18 Influenza Season.
Vaccination is the most effective way to prevent influenza illness and associated complications. For the CDC SUMMARY of Prevention and Control of Influenza with Vaccines 2017–18 Influenza Season.
Annual influenza vaccination is associated with a reduction in influenza-related respiratory illness and physician visits. Hospitalizations and deaths among high risk persons are reduced by annual vaccination. Influenza vaccination has also proven effective in reducing otitis media in children and absenteeism in working adults.
Every year the CDC and the World Heath Organization select influenza A and influenza B strains that have the highest probability of causing a pandemic in the upcoming flu season. These virus strains are then used by vaccine manufacturers to produce the annual vaccine. When the seasonal vaccine is well matched with the actual reassorted strains in circulation, a high inoculation rate can induce herd immunity and limit the spread of epidemics.
The CDC web site identifies: trade name, route, package dosage, age appropriate approved influenza vaccines, single pre-filled syringe of multi-use vial packaging in the following table: Influenza vaccines — United States, 2017–18 influenza season
- Inactivated influenza vaccines (IIVs) are available in both trivalent (IIV3) and quadrivalent (IIV4) formulations.
- Cell culture inactivated influenza vaccine (ccIIV) are available in a quadrivalent formulation (ccIIV4).
- FluMist Quadrivalent (LAIV4; MedImmune, Gaithersburg, Maryland) should not be used during the 2017–18 season due to concerns about its effectiveness against influenza A(H1N1)pdm09 viruses in the United States during the 2013–14 and 2015–16 influenza seasons.
- For the 2018–19 U.S. influenza season, providers may choose to administer any licensed, age-appropriate influenza vaccine (IIV, recombinant influenza vaccine [RIV], or LAIV4). LAIV4 is an option for those for whom it is otherwise appropriate. No preference is expressed for any influenza vaccine product. The Advisory Committee on Immunization Practices will continue to review data concerning the effectiveness of LAIV4 as they become available. Providers should be aware that the effectiveness of the updated LAIV4 containing A/Slovenia/2903/2015 against currently circulating influenza A(H1N1)pdm09-like viruses is not yet known.
Virus strains included in the 2017–18 U.S. vaccines
- Trivalent Influenza Vaccine is A/Michigan/45/2015 (H1N1)pdm09–like virus, an A/Hong Kong/4801/2014 (H3N2)-like virus, and a B/Brisbane/60/2008–like virus (Victoria lineage).
- Quadrivalent influenza vaccines will contain these three viruses and an additional influenza B vaccine virus, a B/Phuket/3073/2013–like virus (Yamagata lineage).
All IIVs contain hemagglutinin (HA) from inactivated virus (killed virus) incapable of replication. The HA is derived from influenza viruses antigenically identical to those recommended for a specific season by the FDA.
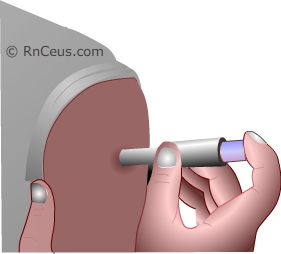 IIVs are delivered via intramuscular (IM) needle injection or prefilled intradermal micro-injection afterwhich the immune system responds
by making antibodies to the viral HA.
IIVs are delivered via intramuscular (IM) needle injection or prefilled intradermal micro-injection afterwhich the immune system responds
by making antibodies to the viral HA.
An intradermal quadrilent IIV is available. It is given into the dermal layer of the skin via a single-dose, prefilled microinjection syringe and that contains less antigen than the intramuscular IIV formulations. The intradermal vaccine was approved for use in people 18 through 64 years of age in 2017-2018.
Live-Attenuated Influenza Vaccine (LAIV) is not recommended for the 2017–18 season, but it is being recommended for the 2018-19 influenza season. On February 21, 2018, Advisory Committee on Immunization Practices (ACIP) recommended that LAIV4 be an option for influenza vaccination of persons for whom it is appropriate for the 2018–19 season. Quadrivalent LAIVs contain two strains of Influenza A and two strains of Influenza B attenuated virus (weakened virus).
LAIVs have been genetically manipulated to replicate efficiently at 25 degrees Celcius and not at all above 39 degrees Celsius. The genetic alteration limits the viral infection to cells in the cooler nasopharynx. LAIVs are manufactured to be delivered by intranasal mist. The immune system responds to the limited nasopharyngeal infection by producing antibodies.
FLU SHOT - IIV (Inactivated influenza vaccine)
|
Who can benefit from the IIV Flu Shot (killed virus):
|
Who Should NOT Get a Flu Shot (killed virus)
|
Who should talk to their doctor before getting the flu shot:
|
Immunizing Health Care Workers
Number of doses for children aged 6 months through 8 years:
© RnCeus.com