Glucose Homeostasis
Most animals, are obliged to catabolize food and use the freed energy to drive anabolic synthesis. In other words, we consume complex substances, break them down to release energy and we use that energy to fuel, build and repair our own cellular components.
From molds to mammals, glucose is quantitatively the most important fuel source for life on earth. It is the primary fuel for our nervous system and the preferred energy source during initial physical activity. Glucose is also an important building block for cellular structures. When the body needs to produce lactose, glycoproteins and glycolipids, they are all synthesized using glucose.
We have two sources of glucose: 1) food, 2) products of metabolism. Food contains carbohydrates, lipids, proteins etc. Dietary carbohydrates are digested to yield simple sugar molecules in the gut. Simple sugars like glucose, galactose and fructose pass from the intestinal lumen to the liver via the portal circulation. Glucose makes up about 80% of absorbed dietary sugars. Galactose and fructose make up the difference.
In addition to dietary carbohydrates, we can synthesize glucose from non carbohydrate products of metabolism (gluconeogenesis). Gluconeogenesis is particularly important during fasting and starvation because the testes, erythrocytes, kidney, lens and cornea are dependant upon glucose as their sole energy source. Glucose is also the primary fuel for the brain but if glucose is low it can use ketone bodies to replace about 20% of the its glucose requirement. Gluconeogenesis can provide the nervous system with a steady supply of glucose even during prolonged fasting.
The activities of daily life require us to consume more nutrients at each meal than we can use immediately. However, the body can store only small quantities of glucose. A total of about 20 grams of glucose are dissolved in the extracellular fluid of an adult. To meet the energy needs between meals and while sleeping the body stores glucose as glycogen or fatty acids.
Adipose, muscle and liver cells are the primary sites of energy storage. Adipose can use glucose to synthesize fatty acids, and triglycerides. Adipose serves as practically unlimited long term storage for energy dense lipids. Muscle stores glucose as glycogen (≈400g) that can be quickly converted back to glucose for muscle activity. The liver can store glucose as glycogen (≈75g) and fatty acids. The liver has the ability to convert stored glycogen to glucose and secrete it into the blood for use by the rest of the body between meals, during fasting and stress. The liver can supply about 16 hours of glucose, after that other energy sources must be mobilized.
In healthy individuals, insulin responsive tissues such as adipose, muscle and liver rapidly absorb glucose to maintain postprandial blood glucose is in a fairly narrow range. In individuals with type I (T1D)and type II (T2D) diabetes, insulin is either absent, deficient or ineffective. Therefore the major actions of insulin including glucose absorption and glucagon suppression are decreased leading to hyperglycemia characteristic of diabetes mellitus. The 2014 American Diabetes Association - Diagnosis and Classification of Diabetes Mellitus:
| Adult values |
Fasting Plasma Glucose |
2-hour post glucose test |
A1C |
| Normal |
<100 mg/dl (<5.6 mmol/l) |
<140 mg/dl (<7.8 mmol/l) |
<5.7% |
| Impaired Fasting Glucose (pre-diabetes) |
100–125 mg/dl (5.6–6.9 mmol/l) |
|
|
| Impaired Glucose Tolerance (pre-diabetes) |
|
140–199 mg/dl (7.8–11.0 mmol/l) |
|
| Diabetes |
≥126 mg/dl (≥7.0 mmol/l) |
≥200 mg/dl (≥11.1 mmol/l) |
≥6.5% |
GLUT |
| GLUT1 |
All cells |
Low affinity/high capacity |
| GLUT2 |
Liver, B-cells, intestine |
Low affinity/high capacity |
| GLUT3 |
Central & peripheral neurons, placenta, testes, platelets |
Low affinity/high capacity |
| GLUT4 |
Striated & cardiac muscle, fat |
insulin and exercise activated |
| GLUT5 |
Mucus membrane, intestine, sperm |
Fructose carrier |
| The rate of glucose diffusion into the cell is dependant upon the concentration of glucose and the number and affinity of transporters in the plasma membrane. |
Glucose homeostasis requires a continuous balance between glucose transport, storage and metabolism. Glucose cannot enter a cell by simple diffusion. The diffusion of glucose across the plasma membrane, must be facilitated by glucose transport (GLUT) molecules. The more GLUT molecules embedded in the plasma membrane, the more glucose can diffuse into the cell. The more glucose that diffuses into cells, the quicker blood glucose level return to normal.
All cells express GLUT 1 in their plasma membrane. It is believed that GLUT 1 is responsible for basal glucose uptake. Some cells like muscle and adipose can translocate additional GLUT 4 molecules from intracellular storage into the plasma membrane in response to insulin. Other cells, like enterocytes in the intestine, can reduce the number of GLUT 2 portals in response to insulin. This has the effect of delaying or reducing dietary glucose absorption. Most cells, importantly the liver and pancreas, can up regulate the gene synthesis and expression of GLUT molecules in response to elevated plasma glucose concentration.
 Intracellular glucose concentration must be controlled. Low intracellular glucose results in stress and starvation. High intracellular levels of glucose have been shown to promote necrotic cell death through H2O2 (peroxide) formation, that may participate in the development of diabetic vasculopathies and associated disease.
Intracellular glucose concentration must be controlled. Low intracellular glucose results in stress and starvation. High intracellular levels of glucose have been shown to promote necrotic cell death through H2O2 (peroxide) formation, that may participate in the development of diabetic vasculopathies and associated disease.
Mammals coordinate four metabolic processes to regulate intracellular glucose, regardless of activity or dietary intake. These processes are: glycolysis, glycogenesis, glycogenolysis, glyconeogenesis.
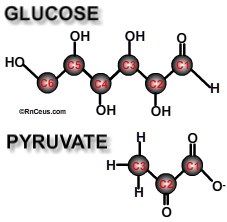
Glycolysis is the enzymatic catabolism of the (6 carbon) sugar glucose into two (3 carbon) molecules of pyruvate + 2ATPs. Glycolysis reduces intracellular glucose that allows additional glucose to safely enter. Glycolysis is a ten-step process. Each step is associated with a particular enzyme catalyst. (Click here for the steps)
Glycogenesis is an anabolic process that requires ATP energy to assemble excess glucose molecules into more complex glycogen granules. A single glycogen granule can contain 30,000 glucose units. Glycogen is synthesized primarily by hepatocytes and muscle. Postprandial glycogen stored in hepatocytes can be as much as 10% of liver mass.
Glycogenesis is initiated by the anabolic hormone insulin. Digestion of food yields glucose that is transported from the gut to the circulating blood. Elevated plasma glucose causes the pancreatic beta cells to release insulin. Increased plasma insulin activates anabolic pathways in the liver, muscle, fat, kidney and brain. These organs react by: 1) facilitating GLUT synthesis, expression and diffusion of glucose into the cell, 2) increasing glucose metabolism, 3) enhancing the storage of excess glucose, 4) inhibiting the catabolic release of glucose by suppressing the action of glucagon, 5) enhancing lipid production, 6) activating gene cascades that up regulate cell growth and differentiation. All of these activities have the effect of reducing excess plasma glucose.
Glycogenolysis is a catabolic process that breaks down stored glycogen into glucose. Glycogenolysis provides glucose during short periods of fasting, between meals, during sleep, etc. Glucagon produced in the pancreatic alpha cell and the adrenal catecholamines epinephrine and norepinephrine are the catabolic hormones that regulate glycogenolysis. Glucagon and catecholamines activate the enzyme glycogen phosphorylase. The liver and muscle are the primary targets of glucagon induced glycogenolysis. Muscle is additionally targeted by catecholamines that are released during stress and strenuous exercise.
Liver and muscle are capable of absorbing large quantities of glucose and storing it as glycogen in response to insulin. Between meals, when plasma insulin is low, glucagon increases glucose availability. Glucagon activates the enzyme glycogen phosphorylase that breaks the bonds holding individual glucose molecules to the glycogen macromolecules stored in hepatocytes and myocytes. In T1D and T2D, glucagon production is not suppressed by insulin. The unopposed action of glucagon in diabetics is responsible at least in part for the characteristic hyperglycemia.
Glucose catabolized from glycogen within a myocyte is used only in that myocyte. Glycogen phosphorylase separates a glucose from glycogen as glucose-1-phosphate. The presence of a phosphate group at the carbon-1 position makes glucose-1-phosphate too large to diffuse out of the myocyte through a GLUT 4 portal. Glucose-1-phosphate must be converted to glucose-6-phosphate to undergo glycolysis. The enzyme phosphoglucomutase removes the phosphate group from the carbon-1 position and adds a phosphate group to the carbon-6 position. Myocytes can then use the available glucose-6-phosphate in glycolysis or return it to storage as glycogen through glycogenesis.
Hepatocytes, unlike myocytes, produce glucose-6-phosphatase. The enzyme glucose-6-phosphatase can hydrolyse glucose-6-phosphate into a phosphate group and a free glucose. A free glucose molecule is small enough to diffuse back to the plasma through a GLUT4 portal. The presence of glucose-6-phosphatase allows the liver to store and release glucose for use by all the cells in the body. Glucose released by the liver is a primary source of energy between meals. It is also a source of hyperglycemia in insulin resistant diabetics.
The catecholamines epinephrine and norepinephrine are hormones secreted by the adrenal medulla. These hormones prepare the body for stress like fight or flight activity. These hormones also activate the enzyme glycogen phosphorylase. Glycogen phosphorylase breaks the bonds holding individual glucose molecules to the glycogen macromolecule. This provides muscle with the fuel needed for quick sprints and heavy lifts.
A recent study by Dufour S. and Lebon V. et al. (2009) demonstrated a 2.5 fold increase of plasma glucose after epinephrine infusion. An inverse association was demonstrated between hepatic glycogen stores and plasma glucose post epinephrine infusion. It was concluded that the majority of hepatic glycogen was converted to glucose in the first 60 minutes following epinephrine infusion and that plasma glucose returned to normal within 90 minutes due to a twofold increase in hepatic glyconeogenesis.
 The liver and kidney express the enzyme glucose-6-phosphatase. These organs use the enzyme glucose-6-phosphatase to remove the phosphate group glucose-6-phosphate. This reaction results in a free glucose molecule and a phosphate group. Free glucose can then diffuse from the cell to blood stream.
The liver and kidney express the enzyme glucose-6-phosphatase. These organs use the enzyme glucose-6-phosphatase to remove the phosphate group glucose-6-phosphate. This reaction results in a free glucose molecule and a phosphate group. Free glucose can then diffuse from the cell to blood stream.
Gluconeogenesis is the synthesis of new glucose from metabolites such as lactate, pyruvate, glycerol, and alanine. For example, lactate formed in muscle and erythrocytes as a metabolic waste product of glycolysis is carried in the blood to the liver where it is converted to glucose and released back to the blood.
- T2D diabetes mellitus increases the rate of gluconeogenesis. Magnusson and colleagues attribute an ≈18% greater fasting glucose production in T2D diabetics to gluconeogenesis.
- T1D diabetes increases hepatic glucose production during exercise and at rest. Peterson and colleagues demonstrated that T1D hyperglycemia at rest and during exercise can be entirely accounted for by an increased gluconeogenesis. Whereas the increased hepatic glucose production during exercise in healthy subjects is the result of glycogenolysis.
- "Administration of Ringer lactate to the diabetic patient undergoing surgery can markedly increase glucose levels. Lactate is a gluconeogenic precursor, and in situations of stress such as surgery the rate of gluconeogenesis may be enhanced." - Joslin's diabetes mellitus
- Certain cancers, especially those with rapid cell growth require large amounts of energy. Glucose production and metabolism is often much higher in these cancer patients than healthy individuals. J. A. Tayek and J. Katz determined that many cancer patients rely on gluconeogenesis for the bulk of their glucose production, while healthy individuals derive less than half their glucose by that means.
- G. Bongaerts and H. van Halteren et al. hypothesize, "In growing tumours the O2 concentration is critically low". "In the absence of sufficient O2 they have to switch to anaerobic dissimilation, with only 2 moles of ATP and 2 moles of lactic acid from 1 mole of glucose". "Therefore, growth of these tumour cells will require about 40 times more glucose than it should require in the presence of sufficient O2". "Compensatory glucose is provided by hepatic gluconeogenesis from lactic acid. However, the liver must invest 3 times more energy to synthesize glucose than can be extracted by tumour cells in an anaerobic way. The liver extracts the required energy from amino acids and especially from fatty acids in an oxidative way. This may account for weight loss, even when food intake seems adequate".
© RnCeus.com
 Intracellular glucose concentration must be controlled. Low intracellular glucose results in stress and starvation. High intracellular levels of glucose have been shown to promote necrotic cell death through H2O2 (peroxide) formation, that may participate in the development of diabetic vasculopathies and associated disease.
Intracellular glucose concentration must be controlled. Low intracellular glucose results in stress and starvation. High intracellular levels of glucose have been shown to promote necrotic cell death through H2O2 (peroxide) formation, that may participate in the development of diabetic vasculopathies and associated disease. The liver and kidney express the enzyme glucose-6-phosphatase. These organs use the enzyme glucose-6-phosphatase to remove the phosphate group glucose-6-phosphate. This reaction results in a free glucose molecule and a phosphate group. Free glucose can then diffuse from the cell to blood stream.
The liver and kidney express the enzyme glucose-6-phosphatase. These organs use the enzyme glucose-6-phosphatase to remove the phosphate group glucose-6-phosphate. This reaction results in a free glucose molecule and a phosphate group. Free glucose can then diffuse from the cell to blood stream.