The
Cell Cycle
Normal cells and abnormal (cancer) cells must complete the cell cycle in order
to replicate. The cell cycle is an ordered sequence of cellular events regulated by chemical signals produced by genes. Some genes (proto-oncogenes) are responsible for stimulating cellular growth and division. Other genes (tumor suppressor genes) restrict cell cycle progression.
In many cases, genetic damage causes cells to lose their ability to produce or respond to the products of tumor suppressor genes. Such cells may embark on an uninhibited cycle of cell replication. Improved understanding of the cell cycle has allowed researchers to develop treatments that can inhibit each specific phase in the cycle and in some cases cause cells to self-destruct (apoptosis).
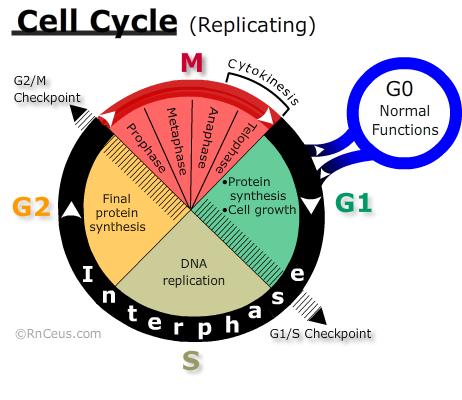 The CELL CYCLE diagram on the right depicts major landmarks on a cells journey to replication. Traditionally the eukaryotic cell cycle is divided into two alternating states; interphase and Mitosis. Shortly after mitosis, internal and external factors determine one of two outcomes for each daughter cell: rest or proliferation (replication). The typical human cell cycle is about 24 hours in length. The G1 phase can last about 11 hours, S phase about 8 hours, G2 phase about 4 hours, and M about 1 hour (Cooper 2000).
The CELL CYCLE diagram on the right depicts major landmarks on a cells journey to replication. Traditionally the eukaryotic cell cycle is divided into two alternating states; interphase and Mitosis. Shortly after mitosis, internal and external factors determine one of two outcomes for each daughter cell: rest or proliferation (replication). The typical human cell cycle is about 24 hours in length. The G1 phase can last about 11 hours, S phase about 8 hours, G2 phase about 4 hours, and M about 1 hour (Cooper 2000).
- Resting cells are considered to be outside of the proliferative cell cycle. Resting cells include two groups. The first group is differentiated cells that carry out all the normal functions of their cell type but do not engage in the activities required for proliferation. The second group, (e.g., stem cells) may be induced to differentiate or proliferate.
- Proliferating cells are those engaged in activities of replication. If they complete all phases of the cell cycle from G1 to mitosis the result is two daughter cells.
Interphase is the part of the cell cycle that produces the intracellular contents necessary to the production of a viable daughter cell. During interphase (G1, S, G2), the sequential progression from one phase to the next is associated with the rise and fall of cyclins and the activation of their cyclin-dependent kinases (CDK) (Tkacs 2020).
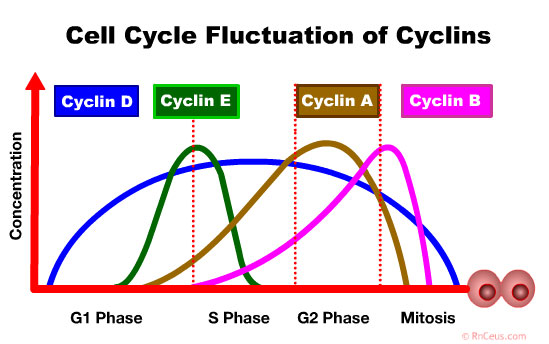 Cyclin D accumulates in the G1 Phase. It drives progression from the G0 or G1 phase into the S phase and is often hyperactivated in many cancers:
Cyclin D accumulates in the G1 Phase. It drives progression from the G0 or G1 phase into the S phase and is often hyperactivated in many cancers:
- Papillary thyroid carcinoma
- Mantle cell lymphoma
- Breast cancer (Stamatakos 2010).
- Cyclin E accumulates at the G1-S phase boundary and is degraded as cell progresses through S phase. Overexpression of this gene results in chromosome instability, and thus may contribute to tumorigenesis in
- Breast cancer
- Ovarian Cancer
- Gastric cancer
- Colorectal carcinomas
- Melanomas
- Non small cell lung carcinoma (Stamatakos 2010).
- Cyclin A is involved in phases S, G2, early M. It is implicated in DNA replication and mitotic entry (Silva Cascales 2021). Guo et al. found high expression of cyclin A2 correlates with a better prognosis in patients with colorectal carcinoma. Conversly, low levels of cyclin A2 was found to induce DNA damage, inflammation, and activation of different regenerative pathways triggering dysplasia in the colonic epithelial cells of mice.
- Cyclin B expression is critical to the initiation of mitosis. It is involved in the disintegration of the nuclear membrane, assembly of the spindle apparatus and segregation of chromosomes (Petry 2016).
Interphase contains several checkpoints that ensure that the cell is competent to complete mitosis. If the cell does not meet the molecular and biologic requirements of a checkpoint, it may not progress beyond the checkpoint. Cells that are blocked at a checkpoint may be triggered to enter apoptosis.
- (G0) – In this "resting" phase, cells are carrying out their normal metabolic functions. They are not replicating and therefore not in cell cycle. Depending on the type of cell, this period can last for a few hours or until the cell dies. Some cells like terminally differentiated muscle cells and most nerve cells remain in G0 indefinitely. In the case of peripheral T-lymphocytes, most are in the G0 phase until they are induced to replicate by contact with an antigen presenting cell. When a G0 cell is signaled to replicate, it moves
into the G1 phase.
- (G1) – During G1 phase, the cell responds to external signals including growth factors by synthesizing the cytoplasmic contents needed for two daughter cells.
The cell will only pass the checkpoint if it is an appropriate size and has adequate energy reserves. At this point, the cell also checks for DNA damage. A cell that does not meet all the requirements will not progress to the S phase. G1 phases cells that do not progress to the S phase can delay progression to correct errors or enter G0.
The G1 phase usually lasts about 11 hours.
- G1/S checkpoint blocks cell-cycle proliferation until external growth factors and available nutrients induce production of sufficient activated cyclin-D+CDK 4 & 6 complexes. Cyclin-D+CDK 4 & 6 complexes release the retinoblastoma protein (Rb) from E2F transcription factors of the G1/S checkpoint. E2Fs are critical regulators of the cell cycle. They regulate every phase of the cell cycle by controlling the transcription of numerous target genes involved in DNA replication and cell cycle progression (Xie 2021).
- In a majority of human cancers, RB1 function is suppressed during tumor progression (Linn 2021).
- G1 phase-specific chemotherapeutic agents include: L-asparaginase and corticosteroids.
- (S) – In S phase, the cell "begins the enormous task of duplicating each of the 3.2 billion base pairs that make up its DNA. Massive replication factories scattered throughout the genome are activated and begin unwinding the double helix and building new DNA molecules at a blistering rate of 500 nucleotides per minute with an error rate of one nucleotide in a billion. In prokaryotes, replication begins from a single site and continues until it terminates at the end of the genome. If eukaryotes were to use an identical strategy, however, it would take on the order of several days to completely replicate its significantly larger genome. Therefore, eukaryotic cells initiate replication from multiple locations referred to as replication origins through out each chromosome (Takeda 2005)."
- Daughter DNA
is replicated (Click) from each parent template DNA strand. Upon completion of the S phase, each chromosome is composed of two DNA double-helix molecules joined at a centromere, forming a chromatid. The S phase lasts about 8 hours.
- S phase-specific agents include: azacitidine, cytarabine, decitabine, doxorubicin, fludarabine, 5-fluorouracil, gemcitabine, hydroxyurea, mercaptopurine. methotrexate, procarbazine, thioguanine.
- (G2) - This phase involves production of additional protein, RNA and the mitotic spindle necessary for mitosis. G2 lasts about 4 hours
- G2/M checkpoint restricts progression to M phase if errors are found in the duplicated chromosomes. Significant errors may trigger apoptosis
- G2 phase-specific agents include: bleomycin, etoposide, irinotecan, topotecan.
- Mitosis (M) Mitosis is the biological sum of four phases: Prophase, Metaphase, Anaphase and Telophase. When mitosis is complete, genetically equivalent chromosomes exists at opposing poles of the cell. Cytokinesis divides the mother cell into two daughter cells. The M phase usually lasts about one hour.
- M phase-specific agents include: docetaxel, paclitaxel, vinblastine, vincristine, vinorelbine, ixabepilone, estramustine
Many chemotherapy agents are cell cycle specific (CCS) drugs
that exert their major cytotoxic effect during a specific
phase of proliferation. Typically CCS drugs have less effect on cells in the resting
state (GO). CCS drugs are administered in the minimum effective concentration via continuous dosing methods rather bolus to maximize the time exposure of cells within the continuum of the targeted phase.
Cell cycle non-specific
(CCNS) drugs like cyclophosphamide or platinum based cisplatin act against cells in all phases of the cell cycle, including the GO
or resting phase. CCNS drugs are dose dependent (>dose = >kill rate). They are most effective and least toxic when given in
high dose bolus. Large solid tumors with a greater fraction
of cells in resting cells may be treated more effectively by CCNS agents.
INSTANT FEEDBACK:
During which phase
is DNA replicated?
Cell
kill theory proposes that a set percentage of cells are killed with each dose
of chemotherapy. The percentage of cells killed depends on the specific drugs
used. For example, if a tumor has 1,000,000 cells and is exposed to a drug that
has a 80% tumor cell kill rate, the first chemotherapy dose will kill 80% or 800,000
of the cancer cells. The second dose will kill another 80% of the cells remaining.
Because only a percentage of cells die with each exposure to a cytotoxic agent,
additional doses of chemotherapy must be repeated to reduce the cancer cells to
just a few remaining cells. When only a few cancer cells remain, it is hoped that
the body’s immune response will then kill the final cells.
Cell cycle time refers to the amount of time required for a cell to move from one
mitosis or cell division to another mitosis. The length of the total cell cycle
varies with the specific type of cell. How long a cell is in the GO or resting
phase is the major factor in the cell cycle time.
Growth
fraction of tumor refers to the percentage of cells engaged in proliferation versus G0 phases at any
given point in time.
Tumor
burden refers to the number of cancer cells present in the tumor. Cancers
with a small tumor burden are usually more sensitive to cytotoxic therapy because
they have a high number of cells reproducing. As the tumor burden increases, the
growth rate slows, and the numbers of cells actively dividing slows down. |
The body has a sophisticated
system for maintaining normal tissue repair and growth. It is thought that the
body responds to a feedback system that signals a G0 (stem cell) to enter the G-1 phase
of the cell cycle in response to tissue damage. In cancer,
this feedback system doesn’t work normally and cancer cells enter the cell
cycle independent of the body’s feedback system. Cancer is a disease
in which cells don’t respond to the normal mechanisms that control normal
cell replication and death. Four characteristics of cancer cells that are not found
in normal cells include:
- uncontrolled cell replication,
- decreased cell differentiation
(the maturation of cells into separate functions),
- the ability to invade
surrounding tissues, and
- the ability to metastasize to other organs and tissues.
INSTANT
FEEDBACK:
The
cell kill theory proposes that a set percentage of cells are killed with
each dose of chemotherapy.
INSTANT
FEEDBACK:
Cancers with a small tumor burden are more sensitive to cytotoxic drugs than tumors with a large number of cells.
Reference
Caudron-Herger, M., Diederichs, S. (2021). Insights from the degradation mechanism of cyclin D into targeted therapy of the cancer cell cycle. Sig Transduct Target Ther 6, 311. https://doi.org/10.1038/s41392-021-00732-y
Cooper GM. The Cell: A Molecular Approach. 2nd edition. Sunderland (MA): Sinauer Associates; 2000. The Eukaryotic Cell Cycle. Available from: https://www.ncbi.nlm.nih.gov/books/NBK9876/
Dumina, M., Zhgun, A., Pokrovskaya, M., Aleksandrova, S., Zhdanov, D., Sokolov, N., & El'darov, M. (2021). A Novel L-Asparaginase from Hyperthermophilic Archaeon Thermococcus sibiricus: Heterologous Expression and Characterization for Biotechnology Application. International journal of molecular sciences, 22(18), 9894. https://doi.org/10.3390/ijms22189894
Eisenthal, A., Eytan, K., Brazowski, E., Gitstein, G., Greenberg, R., & Skornick, Y. (2009). Effects of 5-fu on DNA synthesis and cytotoxicity of human lymphocytes induced by IL-2, TGF-β3 and PGE2. Anticancer Research. Retrieved January 27, 2022, from https://ar.iiarjournals.org/content/29/10/3925
Ghelli Luserna Di Rorà, A., Ghetti, M., Ledda, L. et al.(2021) Exploring the ATR-CHK1 pathway in the response of doxorubicin-induced DNA damages in acute lymphoblastic leukemia cells. Cell Biol Toxicol (2021). https://doi.org/10.1007/s10565-021-09640-x
Guo, Y., Gabola, M., Lattanzio, R., Paul, C., Pinet, V., Tang, R., Turali, H., Bremond, J., Longobardi, C., Maurizy, C., Costa, Q. D., Finetti, P., Boissière-Michot, F., Rivière, B., Lemmers, C., Garnier, S., Bertucci, F., Zlobec, I., Chebli, K., … Hahne, M. (2021). Cyclin A2 maintains colon homeostasis and is a prognostic factor in colorectal cancer. The Journal of Clinical Investigation. Retrieved January 26, 2022, from https://www.jci.org/articles/view/131517
Krall, A., Xu, S., Graeber, T. et al. (2016). Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat Commun 7, 11457. https://doi.org/10.1038/ncomms11457
Linn, P., Kohno, S., Sheng, J., Kulathunga, N., Yu, H., Zhang, Z., Voon, D., Watanabe, Y., & Takahashi, C. (2021). Targeting RB1 Loss in Cancers. Cancers, 13(15), 3737. https://doi.org/10.3390/cancers13153737
National Center for Biotechnology Information (2022). PubChem Pathway Summary for Pathway R-HSA-69620, Cell Cycle Checkpoints, Source: Reactome. Retrieved January 26, 2022 from https://pubchem.ncbi.nlm.nih.gov/pathway/Reactome:R-HSA-69620.
Petry S. (2016). Mechanisms of Mitotic Spindle Assembly. Annual review of biochemistry, 85, 659–683. https://doi.org/10.1146/annurev-biochem-060815-014528
Silva Cascales H, Burdova K, Middleton A, Kuzin V, Müllers E, Stoy H, Baranello L, Macurek L, Lindqvist A. (2021). Cyclin A2 localises in the cytoplasm at the S/G2 transition to activate PLK1. Life Sci Alliance. 2021 Jan 5;4(3):e202000980. doi: 10.26508/lsa.202000980. PMID: 33402344; PMCID: PMC7812317.
Stamatakos, M., Palla, V., Karaiskos, I. et al. (2010). Cell cyclins: triggering elements of cancer or not?. World J Surg Onc 8, 111 ). https://doi.org/10.1186/1477-7819-8-111
Tkacs, Nancy C., et al. (2020). Advanced Physiology and Pathophysiology: Essentials for Clinical Practice. Springer Publishing Company.
Xie, D., Pei, Q., Li, J., Wan, X., & Ye, T. (2021). Emerging role of E2F family in cancer stem cells. Frontiers. Retrieved January 29, 2022, from https://doi.org/10.3389/fonc.2021.723137
©RnCeus.com
 The CELL CYCLE diagram on the right depicts major landmarks on a cells journey to replication. Traditionally the eukaryotic cell cycle is divided into two alternating states; interphase and Mitosis. Shortly after mitosis, internal and external factors determine one of two outcomes for each daughter cell: rest or proliferation (replication). The typical human cell cycle is about 24 hours in length. The G1 phase can last about 11 hours, S phase about 8 hours, G2 phase about 4 hours, and M about 1 hour (Cooper 2000).
The CELL CYCLE diagram on the right depicts major landmarks on a cells journey to replication. Traditionally the eukaryotic cell cycle is divided into two alternating states; interphase and Mitosis. Shortly after mitosis, internal and external factors determine one of two outcomes for each daughter cell: rest or proliferation (replication). The typical human cell cycle is about 24 hours in length. The G1 phase can last about 11 hours, S phase about 8 hours, G2 phase about 4 hours, and M about 1 hour (Cooper 2000). Cyclin D accumulates in the G1 Phase. It drives progression from the
Cyclin D accumulates in the G1 Phase. It drives progression from the