Renal Mechanism of the Bicarbonate Buffer System
The respiratory and the renal systems work together to maintain a stable internal acid-base balance and blood pH. The respiratory system can act quickly (within seconds to minutes) to remove CO2 by increasing the rate of ventilation. CO2 is a key component in the production of metabolic acid (H+).
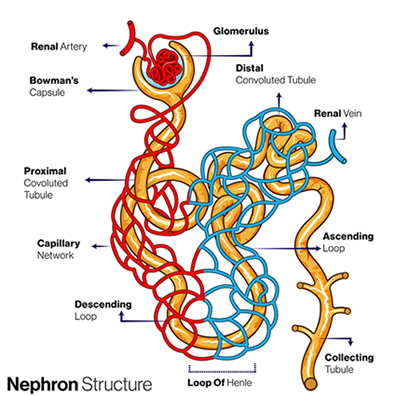 The kidneys, on the other hand, require more time (hours to days) to effect a change in pH. They regulate the pH by reclaiming bicarbonate ( HCO₃⁻) from the glomerular filtrate and return it to the bloodstream. Bicarbonate acts as a buffer in the blood to neutralize acids.
The kidneys, on the other hand, require more time (hours to days) to effect a change in pH. They regulate the pH by reclaiming bicarbonate ( HCO₃⁻) from the glomerular filtrate and return it to the bloodstream. Bicarbonate acts as a buffer in the blood to neutralize acids.
- Acids are molecules or ions that can donate a proton (H+) to another molecule or ion in a solution, thereby increasing the concentration of hydrogen ions and lowering the pH of the solution.
- Bases are molecules or ions that can accept protons (H+) from another molecule or ion in a solution. This acceptance of H+ reduces the concentration of free hydrogen ions, raising the pH of the solution and making it more basic.
- Bicarbonate Reabsorption: In the proximal tubule, most of the filtered bicarbonate (HCO₃⁻) is reabsorbed back into the bloodstream. This helps maintain a readily available buffer system in the blood.
- Ammonia Production: Proximal tubule cells also generate ammonia (NH3) from glutamine, an amino acid.
- Ammonia and Acid Trapping: Ammonia combines with a hydrogen ion (H+) to form ammonium
(NH4+). This effectively traps the H+ within the filtrate, preventing it from re-entering the bloodstream.
- H+- ATPase Pump: In the distal tubule and collecting ducts, H+- ATPase pumps actively transport additional hydrogen ions (H+) from the bloodstream into the filtrate. These H+ ions can then:
- Combine with filtered citrate or phosphate buffers in the filtrate, further removing them from the blood.
- Combine with the previously formed ammonium (NH4+) to form ammonium chloride (NH4Cl), a readily excretable salt.
Chronic kidney disease (CKD) damages nephrons, impairing their ability to reabsorb bicarbonate (HCO₃⁻) and reduce ammonia production.
- Reduced Capacity for Reabsorption:
- As CKD progresses, nephrons (the functional units of the kidney) become damaged and lose their ability to reabsorb bicarbonate effectively. This leads to increased bicarbonate loss in the urine. Decreased transporter activity:
- Specific transporters in the proximal tubules, like the sodium-bicarbonate cotransporter (NBC1), are responsible for reabsorbing bicarbonate. In CKD, the activity of these transporters can be reduced, further hindering HCO₃⁻ absorption.
- Impaired Acid Secretion:
- Ammonia production: The kidneys normally generate ammonia (NH3) to combine with hydrogen ions (H⁺), forming ammonium (NH₄⁺ ). This "traps" the acid within the filtrate for excretion. In CKD, ammonia production can be reduced, limiting this acid-elimination pathway.
- H⁺- ATPase pump activity: The distal tubules and collecting ducts contain H+- ATPase pumps that actively secrete hydrogen ions into the filtrate. Reduced activity of these pumps in CKD weakens the kidneys' ability to eliminate excess acid.
References
Biga, L. M., Bronson, S., Dawson, S., Harwell, A., Hopkins, R., Kaufmann, J., LeMaster, M., Matern, P., Morrison-Graham, K., Oja, K., Quick, D., Runyeon, J., Oeru, O., & OpenStax. (2019, September 26). 26.4 acid-base balance. Anatomy Physiology. https://open.oregonstate.education/aandp/chapter/26-4-acid-base-balance/
Video:overview of the role of the kidneys in acid-base balance. Merck Manual Professional Edition. (n.d.). https://www.merckmanuals.com/professional/multimedia/video/overview-of-the-role-of-the-kidneys-in-acid-base-balance
 The kidneys, on the other hand, require more time (hours to days) to effect a change in pH. They regulate the pH by reclaiming bicarbonate ( HCO₃⁻) from the glomerular filtrate and return it to the bloodstream. Bicarbonate acts as a buffer in the blood to neutralize acids.
The kidneys, on the other hand, require more time (hours to days) to effect a change in pH. They regulate the pH by reclaiming bicarbonate ( HCO₃⁻) from the glomerular filtrate and return it to the bloodstream. Bicarbonate acts as a buffer in the blood to neutralize acids.