OxyHemoglobin Dissociation Curve
Oxygen Delivery to Tissues
The oxygen dissociation curve (ODC) is a graph depicting the relationship between the partial pressure of oxygen (pO2) and the percentage of hemoglobin saturation. This curve quantifies the affinity of hemoglobin for oxygen. It shows the percentage of hemoglobin that is saturated at a given pO2. We can evaluate the O2 affinity of hemoglobin in a blood sample by identifying the pO2 at which 50% of the hemoglobin is saturated (p50). The normal p50 value for adult humans is 24–28 mmHg, or 26.7 mmHg at pH 7.4 and 37°C.
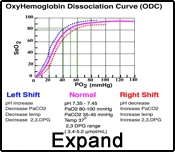 Certain conditions can influence hemoglobin's affinity for O2, causing the curve to be shifted. A left shift means that a p50 will be reached at a lower pO2, indicating increased hemoglobin-oxygen affinity. An alkalotic pH can induce a left shift. Conversely, a decrease in pH (acidosis) can cause the curve to be shifted to the right, denoting a decreased affinity of hemoglobin for oxygen.
Certain conditions can influence hemoglobin's affinity for O2, causing the curve to be shifted. A left shift means that a p50 will be reached at a lower pO2, indicating increased hemoglobin-oxygen affinity. An alkalotic pH can induce a left shift. Conversely, a decrease in pH (acidosis) can cause the curve to be shifted to the right, denoting a decreased affinity of hemoglobin for oxygen.
- The horizontal axis is
Pa02, it represents the partial pressure of oxygen in arterial blood
- The vertical axis is
SaO2, reflects the percentage of hemoglobin binding sites occupied by oxygen.
- Once the PaO2reaches
60 mm Hg the curve flattens, indicating very little change in saturation
that can occur if above PaO2 is increased above this point.
- So, PaO2 of
60 or more is usually considered adequate.
- But, at less than
60 mm Hg the curve is very steep, and small changes in the PaO2
greatly reduce the SaO2.
-
The term "affinity" is used to describe hemoglobin's ability to attract and hold O2 at the heme binding sites.
- Affinity changes
with:
- variation in
pH,
- temperature,
- CO2 and,
- 2,3,-DPG, a metabolic by-product which competes with O2 for binding sites.
- Traditionally the curve starts with:
- pH at 7.4,
- temperature at 37 Centigrade, and
- PaCO2 at 40.
- Changes from these values are called "shifts".
- A left shift will increase oxygen's affinity for hemoglobin.
- In a left shift condition (alkalosis, hypothermia, etc.) oxygen will have a higher affinity
for hemoglobin.
- SaO2 will increase at a given PaO2, but more of it will stay on the hemoglobin and ride back through the lungs without being used. This can result in tissue hypoxia even when there is sufficient oxygen in the blood.
- A right shift decreases oxygen's affinity for hemoglobin.
- In a right shift (acidosis, fever, etc.) oxygen has a lower affinity for hemoglobin.
Blood will release oxygen more readily.
- This means more O2 will be released to the cells, but it also means less oxygen will be carried from the lungs in the first place.
© RnCeus.com
 Certain conditions can influence hemoglobin's affinity for O2, causing the curve to be shifted. A left shift means that a p50 will be reached at a lower pO2, indicating increased hemoglobin-oxygen affinity. An alkalotic pH can induce a left shift. Conversely, a decrease in pH (acidosis) can cause the curve to be shifted to the right, denoting a decreased affinity of hemoglobin for oxygen.
Certain conditions can influence hemoglobin's affinity for O2, causing the curve to be shifted. A left shift means that a p50 will be reached at a lower pO2, indicating increased hemoglobin-oxygen affinity. An alkalotic pH can induce a left shift. Conversely, a decrease in pH (acidosis) can cause the curve to be shifted to the right, denoting a decreased affinity of hemoglobin for oxygen.